Science Away Day on the Strand
May, 2024

Real-time label-free imaging of living crystallization-driven self-assembly
10.26434/chemrxiv-2024-d1gd3
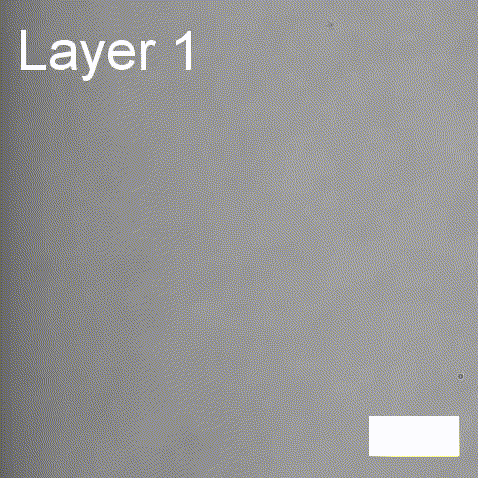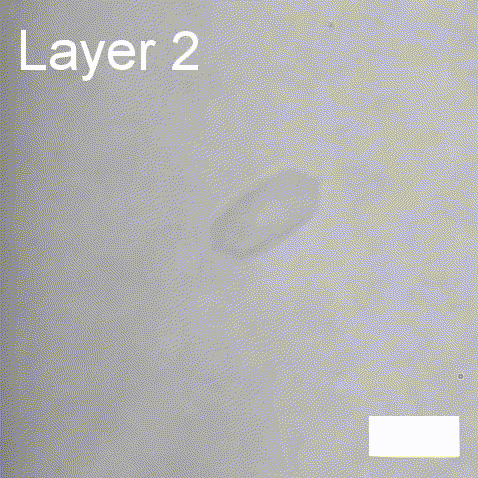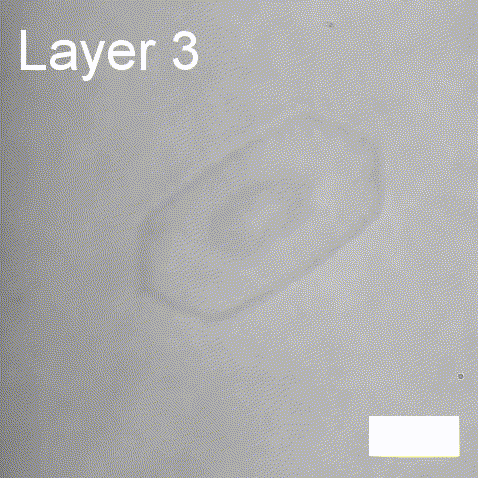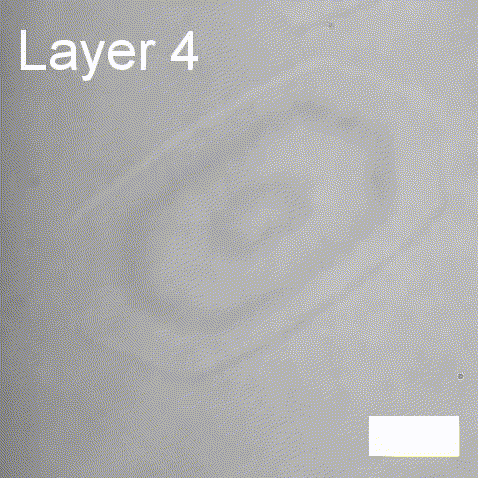
Real-time label-free imaging of living crystallization-driven self-assembly.
The living crystallization-driven self-assembly (CDSA) of semicrystalline block copoly- mers is a powerful method for the bottom-up construction of uniform polymer mi- crostructures with complex hierarchies. Improving our ability to engineer such complex particles demands a better understanding of precisely how to control the self-assembly process. Here, we apply interferometric scattering microscopy (iSCAT) to deliver real- time observation of individual poly(ε-caprolactone)-based platelet growth. This label-free method enables us to map the role of key reaction parameters on platelet growth rate, size, and morphology. Furthermore, iSCAT provides a contrast mechanism for studying multi-layer platelets, offering new insights into the distribution of polymer compositions within a single platelet.
Christmas Dinner in London
December, 2023

Henry Chippindale
October, 2023

Henry Chippindale
Henry joins the group following a BSc in Biology at the University of Nottingham and a Systems and Synthetic Biology MRes from Imperial.
Henry is working on building de novo artifical synapses linking real cells and artificial cells.
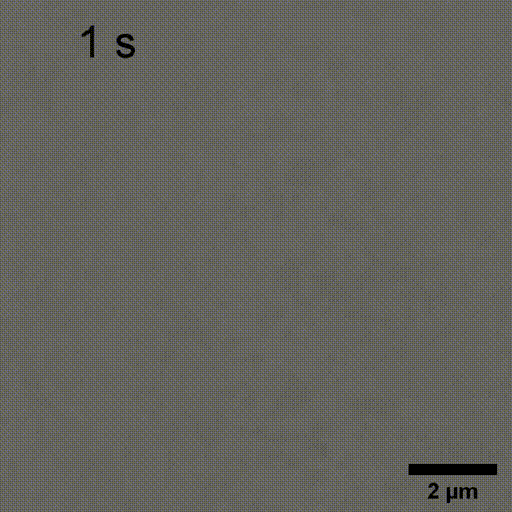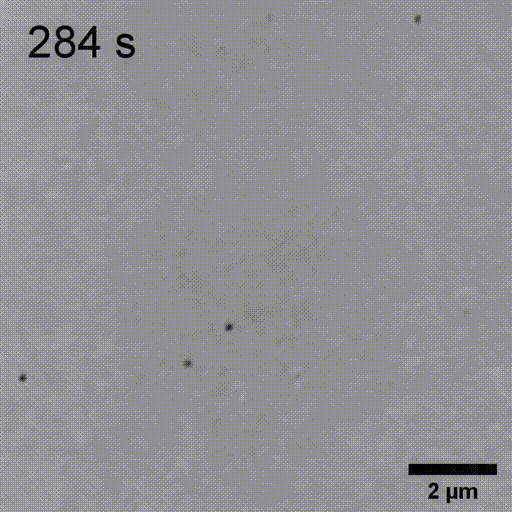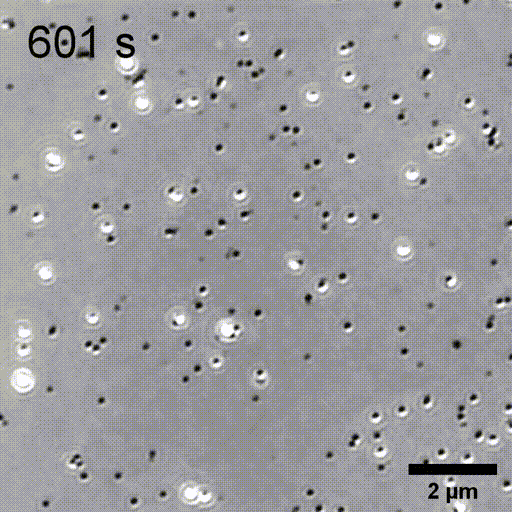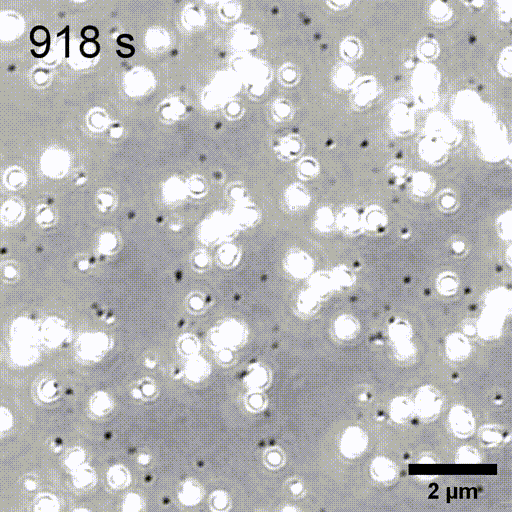
Monitoring AuNP growth.
Methods capable of controlling synthesis at the level of an individual nanoparticle are a key step towards improved reproducibility and scalability in engineering complex nanomaterials. To address this we combine spatially-patterned activation of the photoreductant sodium pyruvate with interferometric scattering microscopy to achieve fast, label-free monitoring and control of hundreds of gold nanoparticles in real-time. Individual particle growth kinetics are well-described by two-step nucleation autocatalysis model, but with a distribution of individual rate constants that changes with reaction conditions.
NanoSeries 2023 Poster Prize
June, 2023

Pantelitsa Dimitriou
March, 2023

Pan Dimitriou
Pantelitsa joined the Wallace Group in March 2023 as a PDRA, after completing her PhD in Engineering. Her research interests lie in 3D printing, microfluidics and cell membrane mimics. During her time in the Wallace group, she will explore the combination of droplet-microfluidics, multiple emulsions and hydrogels in the formation of artificial bacterial envelopes.
Christian Bortolini
January, 2023

Christian Bortolini
Christian joined the Wallace group as a PDRA to work on the membrane attack complex. The main objective of his project is to understand how complement activation kills pathogenic bacteria.
Mudchute Farm
December, 2022

Yize Wu
November, 2022

Yize Wu
joining us as a MRes student in Interdisciplinary Chemistry, Yize joins us to work on biohybrid nanopores.