The TatC component of the twin-arginine protein translocase functions as an obligate oligomer.
10.1111/mmi.13106
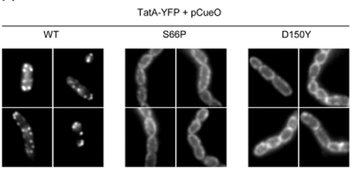
Substrate-induced assembly of TatA-YFP is blocked by TatC mutations.
The Tat protein export system translocates folded proteins across the bacterial cytoplasmic membrane and the plant thylakoid membrane. The Tat system in Escherichia coli is comprised of TatA, TatB and TatC proteins. TatB and TatC form an oligomeric, multivalent receptor complex that binds Tat substrates, while multiple protomers of TatA assemble at substrate-bound TatBC receptors to facilitate substrate transport. We have addressed whether oligomerisation of TatC is an absolute requirement for operation of the Tat pathway by screening for dominant negative alleles of tatC that inactivate Tat function in the presence of wild type tatC. Single substitutions that confer dominant negative TatC activity localised to the periplasmic cap region. The variant TatC proteins retained the ability to interact with TatB and with a Tat substrate but were unable to support the in vivo assembly of TatA complexes. Blue-native PAGE analysis showed that the variant TatC proteins produced smaller TatBC complexes than the wild type TatC protein. The substitutions did not alter disulphide crosslinking to neighbouring TatC molecules from positions in the periplasmic cap but abolished a substrate-induced disulphide crosslink in transmembrane helix five of TatC. Our findings show that TatC functions as an obligate oligomer.