Fast slow folding of an outer membrane porin
10.1073/pnas.2121487119
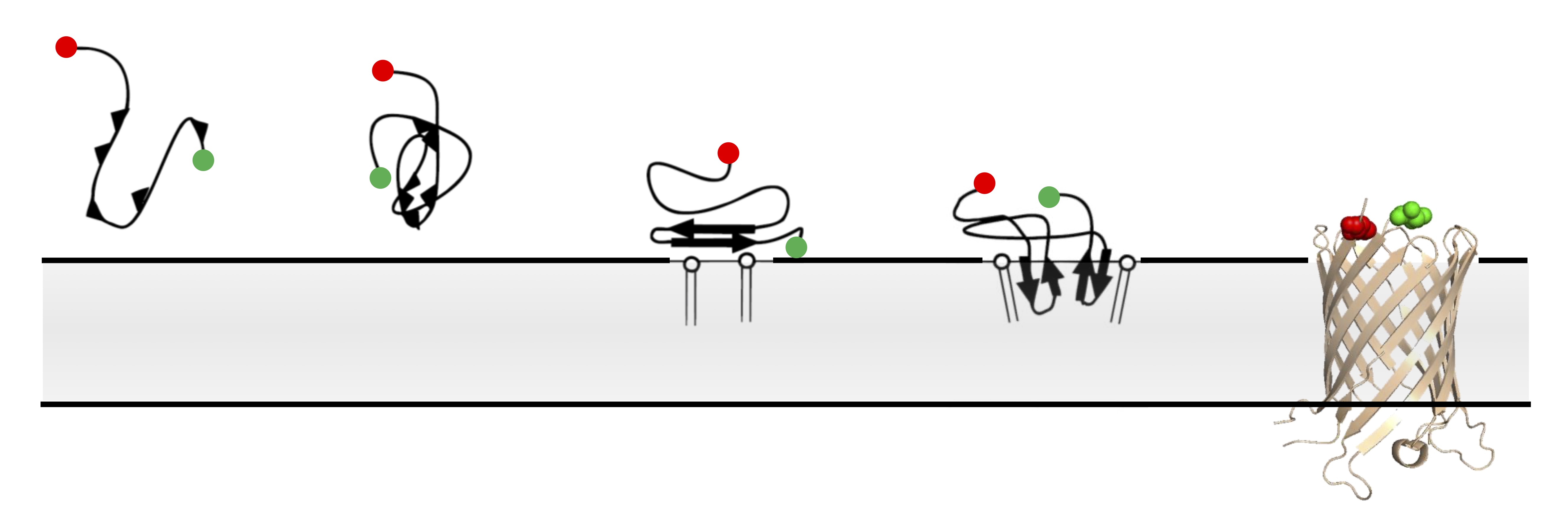
Single-molecule FRET imaging of beta-barrel folding.
In comparison to globular proteins, the spontaneous folding and insertion of β-barrel membrane proteins is surprisingly slow, typically occurring on the order of minutes. Using single-molecule Förster Resonance Energy Transfer to report on the folding of fluorescently-labelled Outer Membrane Protein G we measured the real-time insertion of a β-barrel membrane protein from an unfolded state. Folding events were rare, and fast (<20 ms); occurring immediately upon arrival at the membrane. This combination of infrequent, but rare, folding resolves this apparent dichotomy between slow ensemble kinetics, and the typical timescales of biomolecular folding.