Enhanced stability and fluidity in droplet on hydrogel bilayers for measuring membrane protein diffusion.
10.1021/nl071943y
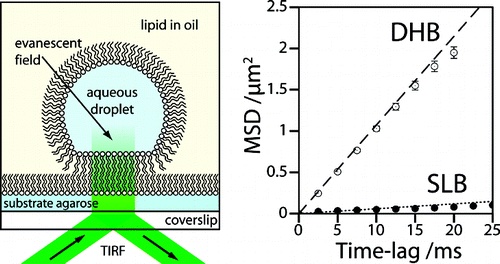
DIBs are good for membrane proteins.
We form artificial lipid bilayers suitable for single-molecule fluorescence microscopy by contacting an aqueous droplet with a hydrogel support immersed in a solution of lipid in oil. Our results show that droplet on hydrogel bilayers (DHBs) have high lipid mobilities, similar to those observed in unsupported lipid bilayers. DHBs are also stable over a period of several weeks. We examine membrane protein diffusion in these bilayers and report a decreased lateral mobility of the heptameric beta-barrel pore-forming toxin alpha-hemolysin versus that of its monomeric precursor. These results corroborate previous models of the alpha-hemolysin insertion mechanism where the monomer binds to the lipid bilayer without insertion.